CSPI asks FDA to ban powdered caffeine sold as a dietary supplement
Por um escritor misterioso
Last updated 21 setembro 2024

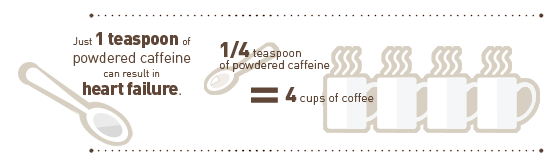
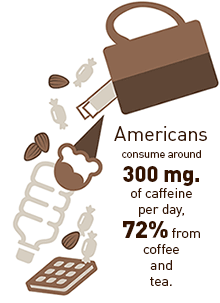
The Center for Science in the Public Interest (CSPI) has sent a petition to federal regulators seeking the ban of pure, powdered caffeine that is packaged and sold as a dietary supplement. Because of the product’s extreme potency, the possibility of accidental overdose poses a clear and present public health risk, the organization asserts.

FDA targets bulk powder caffeine sales in warning letter

Food Safety Marketing News Mérieux NutriSciences
Waking up to the facts about caffeine

Nutrition Page 2There

pure facts archives - Feingold Association
Dietetic Intern Research Articles, Human Sciences
Waking up to the facts about caffeine

caffeine Food Safety News

FDA to explore effect of caffeine on the health of children

caffeine Food Safety News
Recomendado para você
-
 BulkSupplements Whey Protein Isolate Review - How's It Taste?21 setembro 2024
BulkSupplements Whey Protein Isolate Review - How's It Taste?21 setembro 2024 -
 BULKSUPPLEMENTS.COM D-Ribose Powder - Dietary Supplement for Energy & Muscle Support - Unflavored - 5g (5000mg) per Serving, 200 Servings (1 Kilogram - 2.2 lbs) : Health & Household21 setembro 2024
BULKSUPPLEMENTS.COM D-Ribose Powder - Dietary Supplement for Energy & Muscle Support - Unflavored - 5g (5000mg) per Serving, 200 Servings (1 Kilogram - 2.2 lbs) : Health & Household21 setembro 2024 -
 HARDCORE BULK Muscle Growth - Optimal Nutrition & Supps21 setembro 2024
HARDCORE BULK Muscle Growth - Optimal Nutrition & Supps21 setembro 2024 -
 Chamomile Extract21 setembro 2024
Chamomile Extract21 setembro 2024 -
 Bulk Supplements - USA Official Website21 setembro 2024
Bulk Supplements - USA Official Website21 setembro 2024 -
Who wants over 500+ pure bulk supplements with no fillers or additives, Regret Buying21 setembro 2024
-
 BulkSupplements.com Influencer Program21 setembro 2024
BulkSupplements.com Influencer Program21 setembro 2024 -
 Bulk Supplements & Bulk Vitamins for Nutrition21 setembro 2024
Bulk Supplements & Bulk Vitamins for Nutrition21 setembro 2024 -
 BulkSupplements L-Citrulline DL-Malate 2:1 - 250 Grams21 setembro 2024
BulkSupplements L-Citrulline DL-Malate 2:1 - 250 Grams21 setembro 2024 -
 Best Supplements For Bulking To Grow And Gain Muscles-Health News , Firstpost21 setembro 2024
Best Supplements For Bulking To Grow And Gain Muscles-Health News , Firstpost21 setembro 2024
você pode gostar
-
 Danmachi Season 4 Episode 10 EnglishSub HD - BiliBili21 setembro 2024
Danmachi Season 4 Episode 10 EnglishSub HD - BiliBili21 setembro 2024 -
 Map of Camp Half-Blood - Bookmark 2 W X 6 H inches BOOKMARK Percy Jack – SHOP DisBeans21 setembro 2024
Map of Camp Half-Blood - Bookmark 2 W X 6 H inches BOOKMARK Percy Jack – SHOP DisBeans21 setembro 2024 -
 Análise: Bake 'n Switch (Switch): fofura e frustração lado a lado21 setembro 2024
Análise: Bake 'n Switch (Switch): fofura e frustração lado a lado21 setembro 2024 -
 Heimdall or Rig: One God, Two Names (Plus Controversy) - Owlcation21 setembro 2024
Heimdall or Rig: One God, Two Names (Plus Controversy) - Owlcation21 setembro 2024 -
 Can PC and PS4 Players CrossPlay GTA 5 Online Together Cross Platform21 setembro 2024
Can PC and PS4 Players CrossPlay GTA 5 Online Together Cross Platform21 setembro 2024 -
 Defective mitophagy and synaptic degeneration in Alzheimer's disease: Focus on aging, mitochondria and synapse - ScienceDirect21 setembro 2024
Defective mitophagy and synaptic degeneration in Alzheimer's disease: Focus on aging, mitochondria and synapse - ScienceDirect21 setembro 2024 -
 Pokemon Go Eevee Evolutions: How to evolve to Sylveon, Espeon, Umbreon, Leafeon, Glaceon, Vaporeon, Jolteon & Flareon21 setembro 2024
Pokemon Go Eevee Evolutions: How to evolve to Sylveon, Espeon, Umbreon, Leafeon, Glaceon, Vaporeon, Jolteon & Flareon21 setembro 2024 -
If Asta combined all his swords and changed into full demon form, how powerful will he be? - Quora21 setembro 2024
-
/i.s3.glbimg.com/v1/AUTH_08fbf48bc0524877943fe86e43087e7a/internal_photos/bs/2022/C/I/Y7KBKITJuc6k2LAkIwQw/gettyimages-1263581892.jpg) Você tem bons hábitos de segurança na internet? Faça o teste e21 setembro 2024
Você tem bons hábitos de segurança na internet? Faça o teste e21 setembro 2024 -
 Lords of the Fallen Gamespot Review : r/Asmongold21 setembro 2024
Lords of the Fallen Gamespot Review : r/Asmongold21 setembro 2024
