Comparison of AaL active site with AiiA, AiiB, and AidC. (A)
Por um escritor misterioso
Last updated 15 abril 2025


Structural and Biochemical Characterization of AidC, a Quorum-Quenching Lactonase with Atypical Selectivity. - Abstract - Europe PMC

WO2020185861A1 - Proteins and methods for disrupting bacterial communication - Google Patents
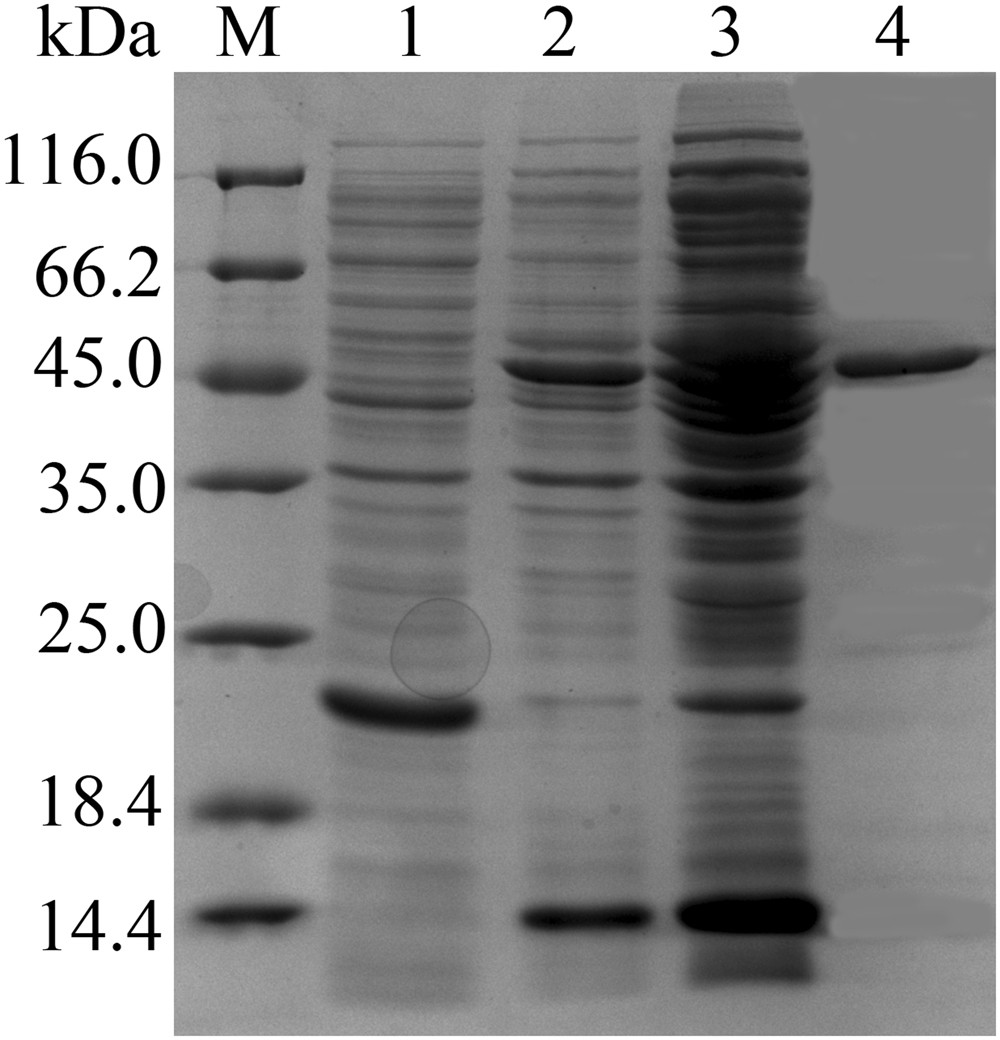
Characterization of AiiK, an AHL lactonase, from Kurthia huakui LAM0618T and its application in quorum quenching on Pseudomonas aeruginosa PAO1

SsoPox W263 saturation site screening and characterization. A. Relative
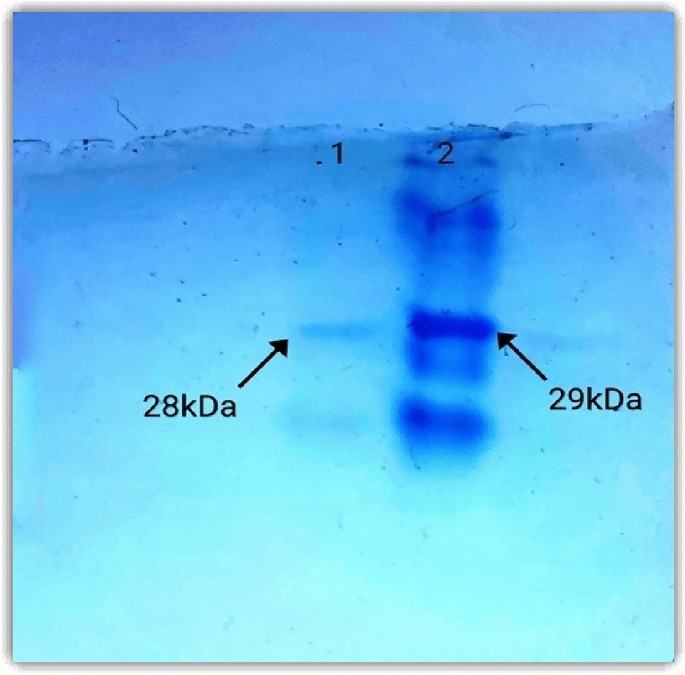
Cloning and characterization of Aiia, an acylhomoserine lactonase from Bacillus cereus RC1 to control soft rot causing pathogen Lelliottia amnigena RCE
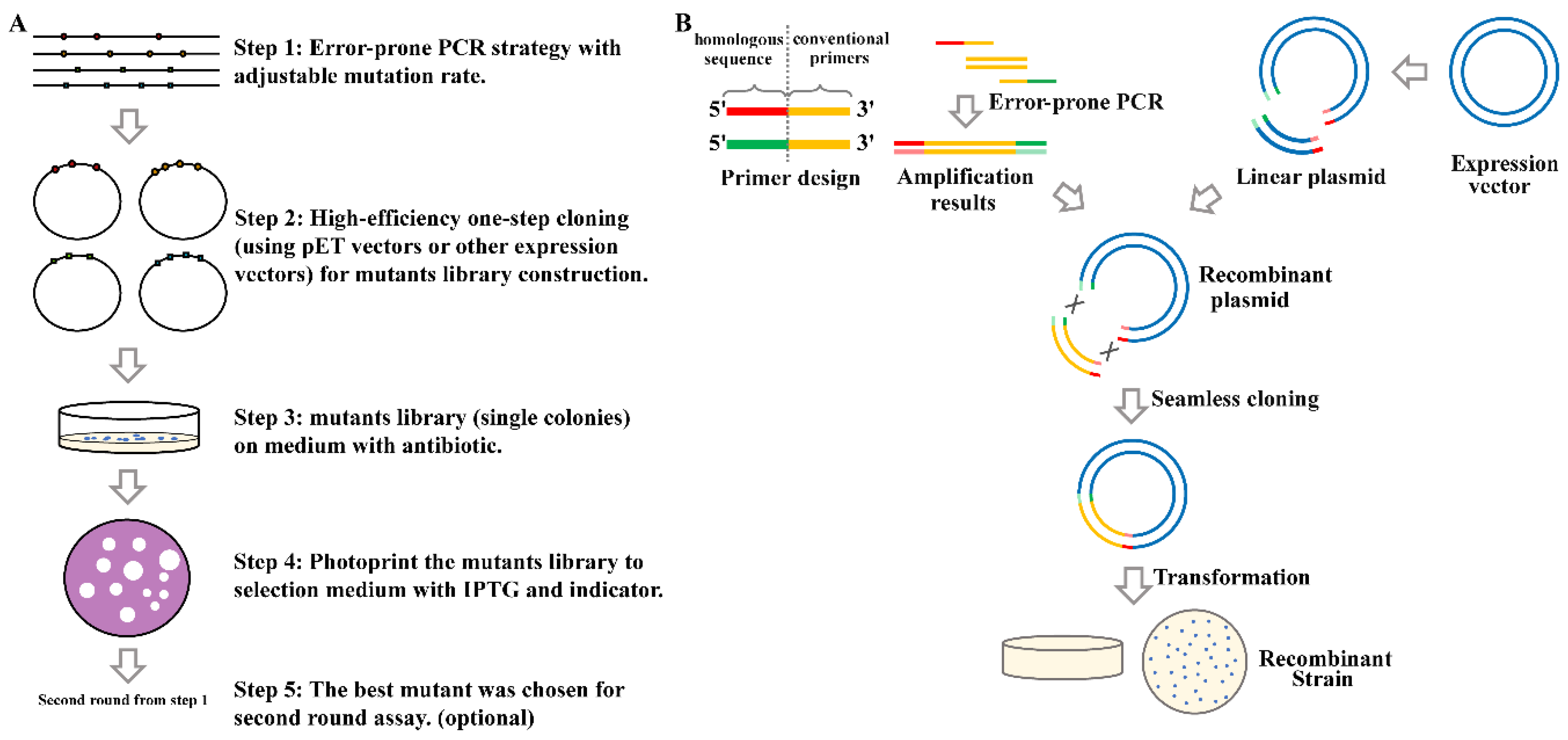
Marine Drugs, Free Full-Text
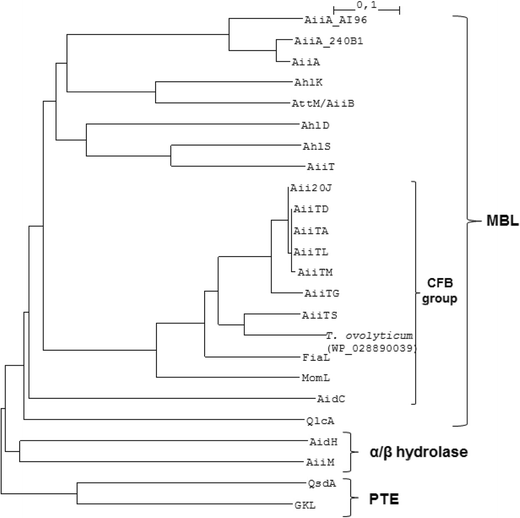
Aii20J, a wide-spectrum thermostable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J, can quench AHL-mediated acid resistance in Escherichia coli

Engineering acyl-homoserine lactone-interfering enzymes toward bacterial control. - Abstract - Europe PMC
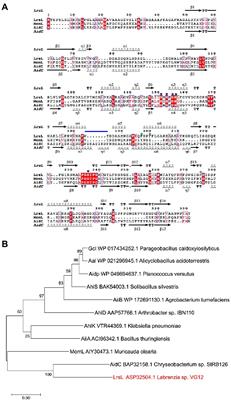
Frontiers The exceptionally efficient quorum quenching enzyme LrsL suppresses Pseudomonas aeruginosa biofilm production

Promiscuous metallo-β-lactamases: MIM-1 and MIM-2 may play an essential role in quorum sensing networks - ScienceDirect

Mechanism of the Quorum-Quenching Lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate Modeling and Active Site Mutations

Lactonase - an overview
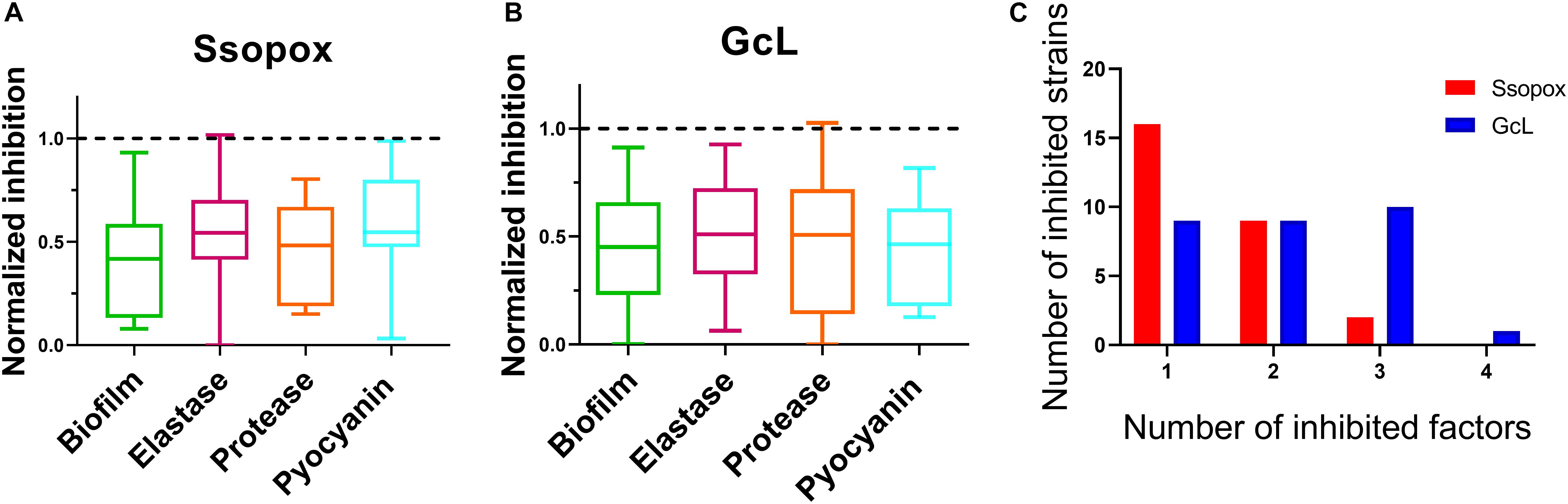
Frontiers Effects of Signal Disruption Depends on the Substrate Preference of the Lactonase

Visualizing the superfamily of metallo-β-lactamases through sequence similarity network neighborhood connectivity analysis - ScienceDirect
Recomendado para você
-
 Trials - POWER UP RUNES - EinHerJar l Myth: Gods of Asgard15 abril 2025
Trials - POWER UP RUNES - EinHerJar l Myth: Gods of Asgard15 abril 2025 -
 Viktor Rydberg — Teutonic Mythology — Contents15 abril 2025
Viktor Rydberg — Teutonic Mythology — Contents15 abril 2025 -
Asgard (@asgard49)'s videos with Originalton - Asgard15 abril 2025
-
 NORROENA VOL III15 abril 2025
NORROENA VOL III15 abril 2025 -
 nwht_2016-11-04 by Shaw Media - Issuu15 abril 2025
nwht_2016-11-04 by Shaw Media - Issuu15 abril 2025 -
Skull song lyrics15 abril 2025
-
 LASER DISTRIBUTOR PTE LTD - VR-Zone15 abril 2025
LASER DISTRIBUTOR PTE LTD - VR-Zone15 abril 2025 -
 Scotts (Clyde)15 abril 2025
Scotts (Clyde)15 abril 2025 -
Adelphi: Blavatsky Publishes The Theosophical Glossary.15 abril 2025
-
 Dante's Hex Pendant by Alchemy Gothic • the dark store™15 abril 2025
Dante's Hex Pendant by Alchemy Gothic • the dark store™15 abril 2025
você pode gostar
-
 Watashi ga Motete Dousunda – 11 – RABUJOI – An Anime Blog15 abril 2025
Watashi ga Motete Dousunda – 11 – RABUJOI – An Anime Blog15 abril 2025 -
 TOME: Immortal Arena Game Review15 abril 2025
TOME: Immortal Arena Game Review15 abril 2025 -
 Galápagos, Telestrations, Jogo de Tabuleiro para Amigos, 4 a 815 abril 2025
Galápagos, Telestrations, Jogo de Tabuleiro para Amigos, 4 a 815 abril 2025 -
Season 1 Episode 5 Preview House of the Dragon (HBO) - video15 abril 2025
-
my dog stepped on a bee rhyme|TikTok Search15 abril 2025
-
 Cartões de Natal Personalizados para Imprimir - 400 Modelos - Gráfica expanSSiva15 abril 2025
Cartões de Natal Personalizados para Imprimir - 400 Modelos - Gráfica expanSSiva15 abril 2025 -
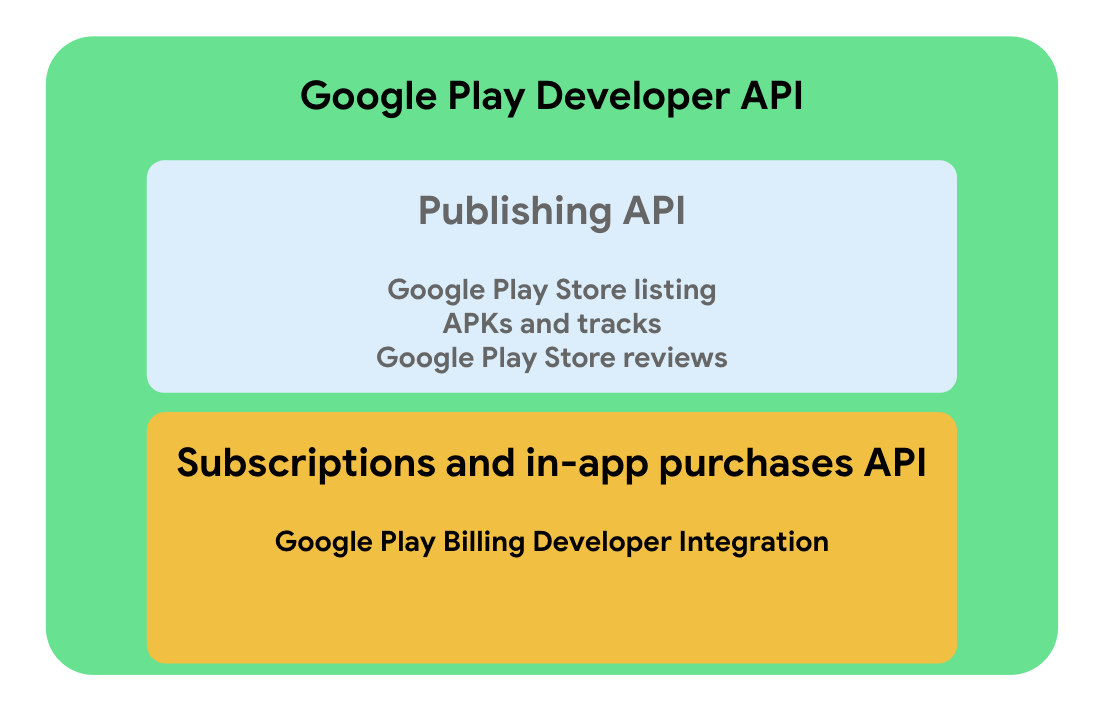 Sistema de faturamento do Google Play15 abril 2025
Sistema de faturamento do Google Play15 abril 2025 -
English Winglish - Word of the day, folks! Did you know the15 abril 2025
-
 Demon Slayer: 3ª temporada terá dublagem pela Crunchyroll - Anime Vício15 abril 2025
Demon Slayer: 3ª temporada terá dublagem pela Crunchyroll - Anime Vício15 abril 2025 -
GitHub - kon172verma/IIT-Delhi-Masters-Thesis: Using the Mass Media data, along with Census and GIS data, we wish to analyze the socio-economic growth in various Indian Districts. Some techniques used: Doc-2-Vec, Hierarchical Clustering, TF-IDF15 abril 2025



