In Vitro vs In Vivo Preclinical Studies
Por um escritor misterioso
Last updated 18 abril 2025
Before a drug candidate can be tested in humans, its safety and efficacy must be explored in in vitro or in vivo preclinical studies.
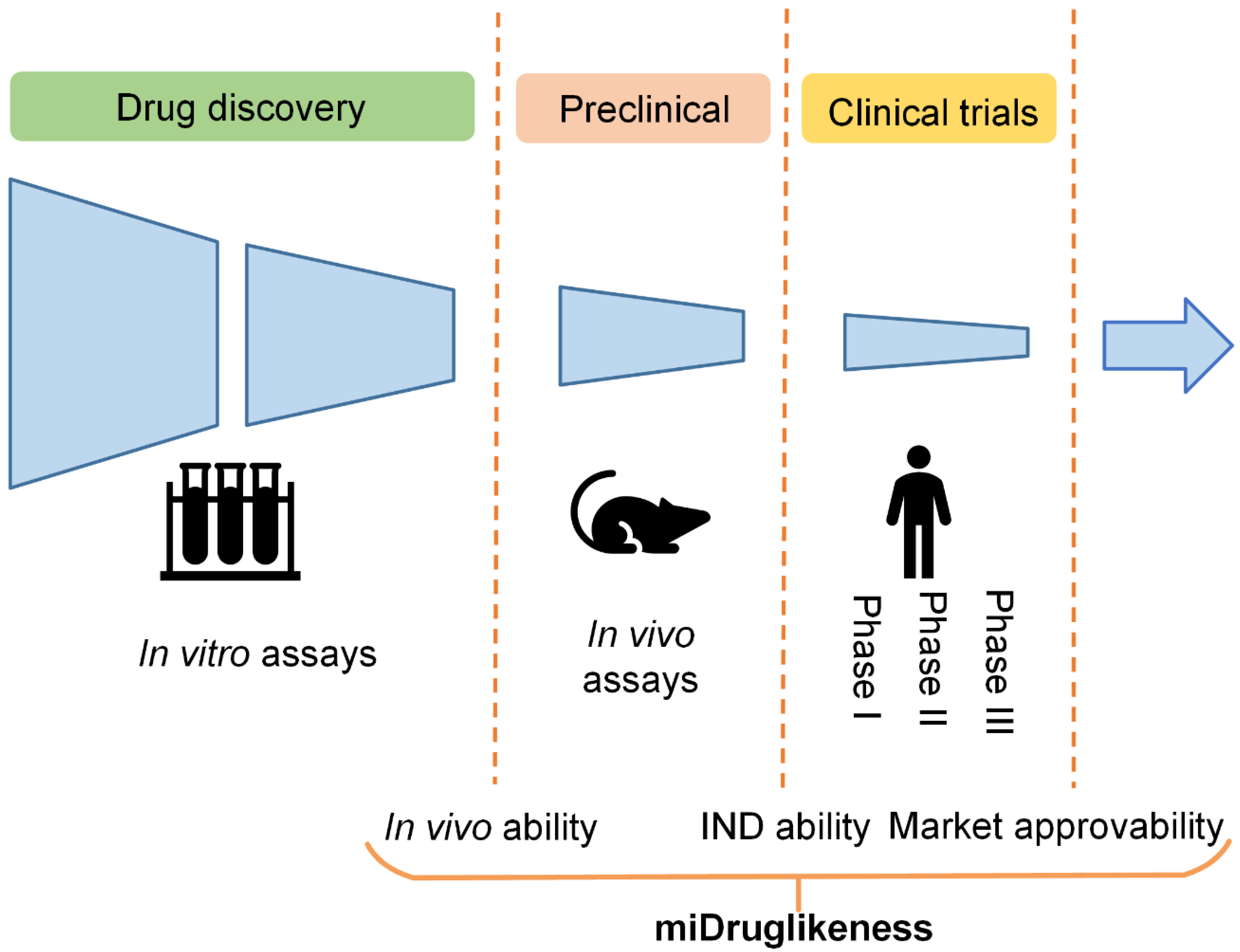
Biomolecules, Free Full-Text
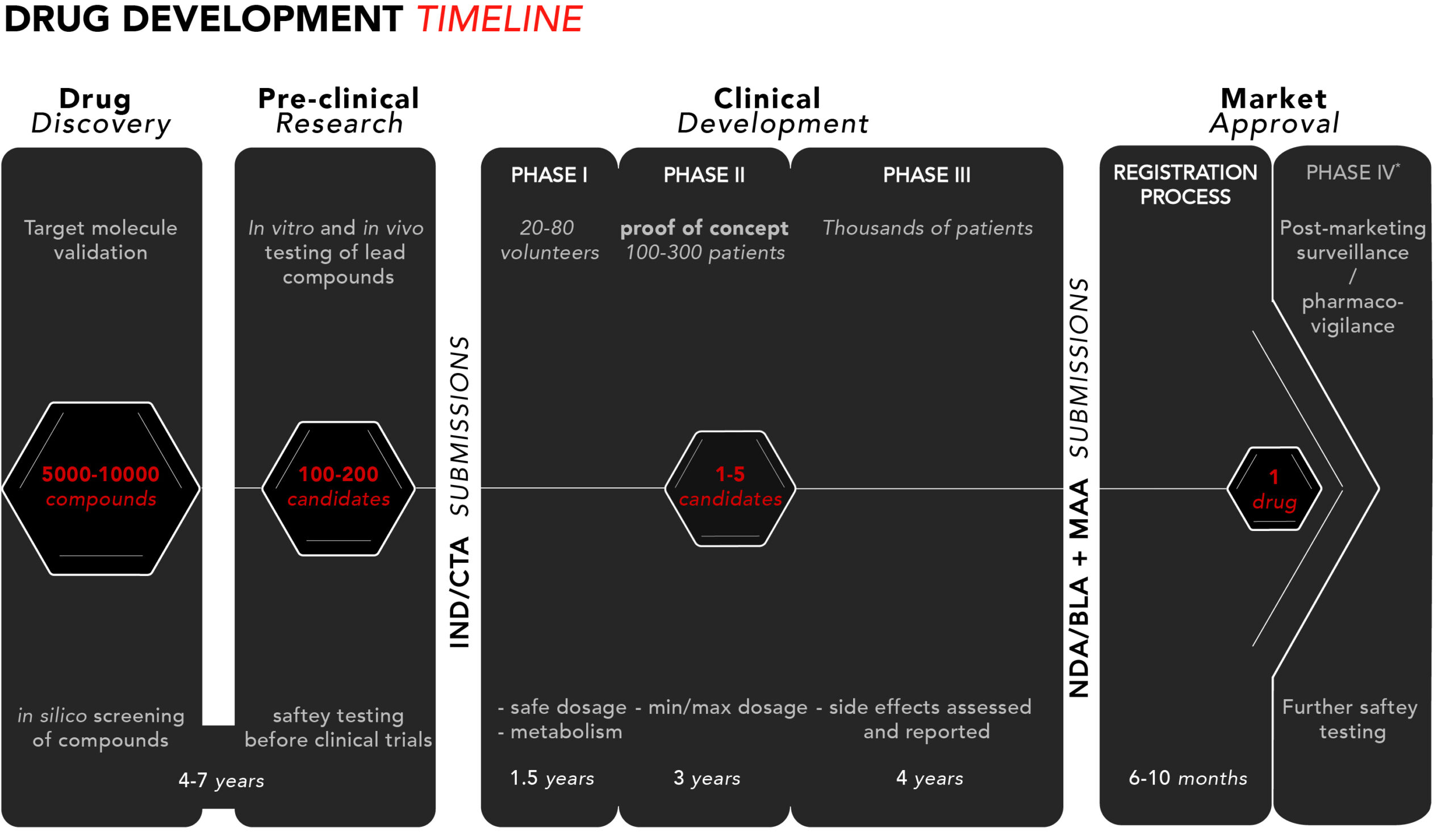
Drug development – The four phases - BioStock

Limitations of Animal Studies for Predicting Toxicity in Clinical

Extrapolation of in vitro data to preclinical and.pptx

Preclinical Animal Studies-Preclinical CRO Medicilon
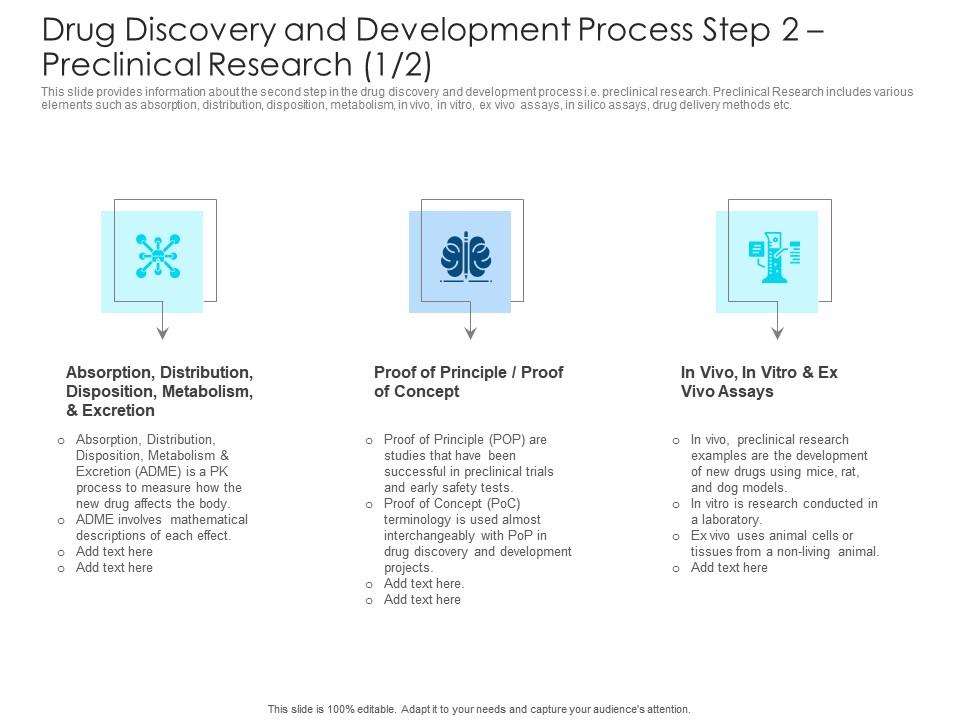
Drug Discovery And Development Process Step 2 Preclinical Research

Use of CRISPR/Cas9 gene editing to improve chimeric antigen
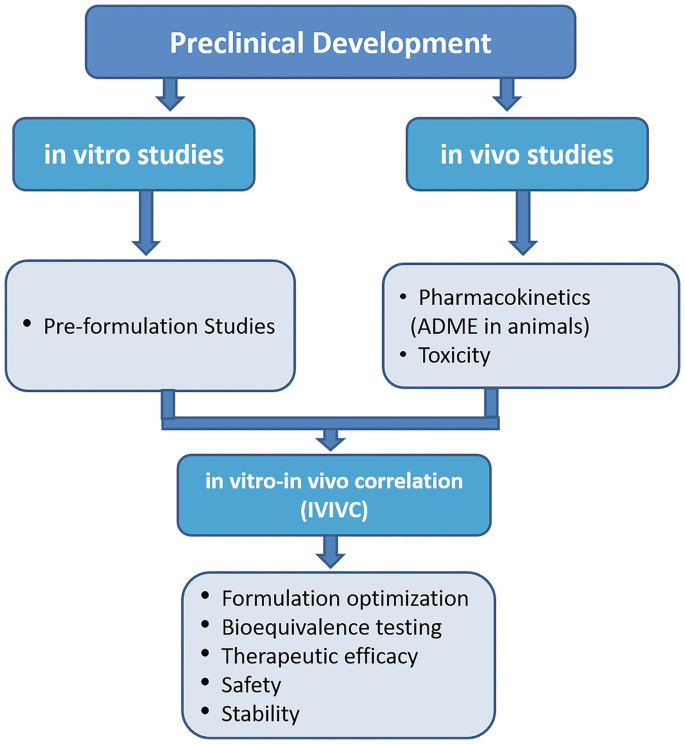
Preclinical In Vivo Drug Development Studies: Limitations, Model
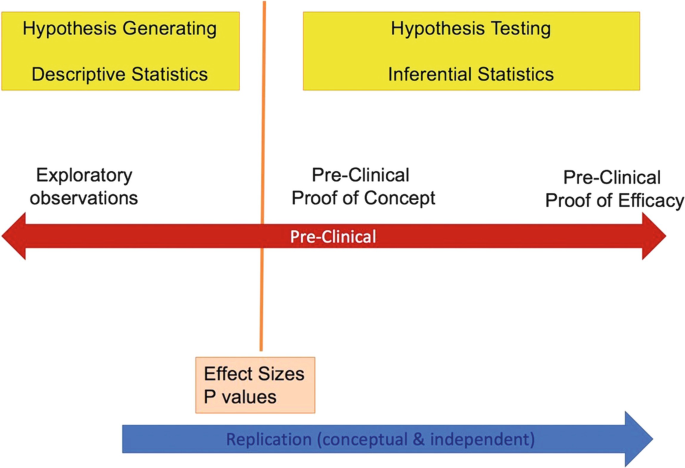
General Principles of Preclinical Study Design

In Vivo Preclinical Imaging - An Essential Tool in Translational

In vitro vs. In vivo: Is One Better?
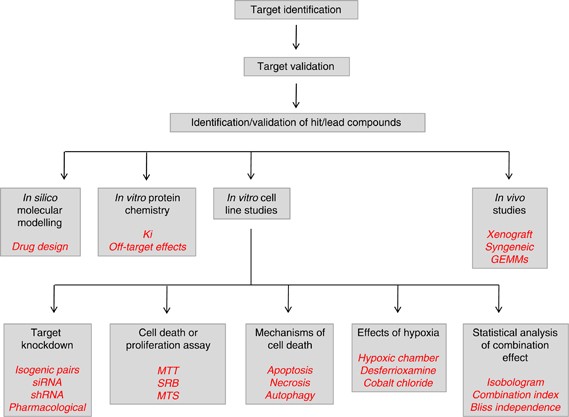
Guidelines for preclinical and early phase clinical assessment of
Recomendado para você
-
 Teste de Velocidade Vivo Fibra ( Teste POWER Vivo )18 abril 2025
Teste de Velocidade Vivo Fibra ( Teste POWER Vivo )18 abril 2025 -
Vivo X90 and MediaTek Dimensity 9200 fail to outperform the A16 Bionic in a real-world gaming test - News18 abril 2025
-
 Biosafety Testing Services18 abril 2025
Biosafety Testing Services18 abril 2025 -
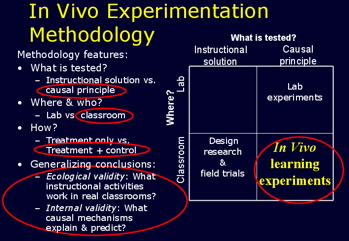 In vivo experiment - LearnLab18 abril 2025
In vivo experiment - LearnLab18 abril 2025 -
 Vivo x60 Pro Plus review: Putting the phone's gimbal camera to the test18 abril 2025
Vivo x60 Pro Plus review: Putting the phone's gimbal camera to the test18 abril 2025 -
 VIVO Y12A Y12S Y20A (PD2060) Test Point (EDL Mod)18 abril 2025
VIVO Y12A Y12S Y20A (PD2060) Test Point (EDL Mod)18 abril 2025 -
 Dimensity 9300, 16 GB RAM and 1 TB storage: Vivo X100 in the performance test!18 abril 2025
Dimensity 9300, 16 GB RAM and 1 TB storage: Vivo X100 in the performance test!18 abril 2025 -
 vivo X90 Pro+ ranks 10th in DxOMark, scores 140 points in global camera test18 abril 2025
vivo X90 Pro+ ranks 10th in DxOMark, scores 140 points in global camera test18 abril 2025 -
 in vivo SPF testing of sunscreens18 abril 2025
in vivo SPF testing of sunscreens18 abril 2025 -
 DLZXWIN TestBox DL S300 3IN1 LCD Screen Tester Machine For iPhone18 abril 2025
DLZXWIN TestBox DL S300 3IN1 LCD Screen Tester Machine For iPhone18 abril 2025
você pode gostar
-
 Um desenho de um alienígena azul com uma cabeça verde e uma cabeça azul.18 abril 2025
Um desenho de um alienígena azul com uma cabeça verde e uma cabeça azul.18 abril 2025 -
 CapCut_soca fofo x soca forte da minha sala18 abril 2025
CapCut_soca fofo x soca forte da minha sala18 abril 2025 -
 Saveiro Titan Turbo - Anúncios para Alta performance18 abril 2025
Saveiro Titan Turbo - Anúncios para Alta performance18 abril 2025 -
 Ravenclaw™ Buffalo Check Headband18 abril 2025
Ravenclaw™ Buffalo Check Headband18 abril 2025 -
 DmC: Devil May Cry - Definitive Edition PlayStation 4 Box Art Cover by AgentLampshade18 abril 2025
DmC: Devil May Cry - Definitive Edition PlayStation 4 Box Art Cover by AgentLampshade18 abril 2025 -
My ROM Hack Showcase of Pokémon Eris Emerald! What do you think of18 abril 2025
-
 Guys, How can i get free GTA SA ? If anybody can help me with that, I'd be very grateful to you. I'm broke i need help : r/GTA18 abril 2025
Guys, How can i get free GTA SA ? If anybody can help me with that, I'd be very grateful to you. I'm broke i need help : r/GTA18 abril 2025 -
 Coloring page - Carro do vintage18 abril 2025
Coloring page - Carro do vintage18 abril 2025 -
 Bitlife Online18 abril 2025
Bitlife Online18 abril 2025 -
 Form Battle: KANTO vs. ALOLA POKÉMON (Pokémon Sun/Moon)18 abril 2025
Form Battle: KANTO vs. ALOLA POKÉMON (Pokémon Sun/Moon)18 abril 2025
